Description
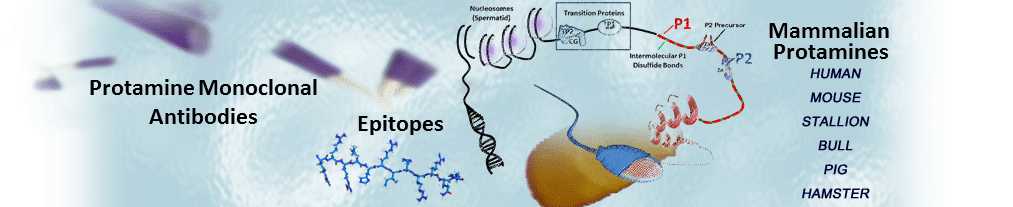
These antibodies were developed at Lawrence Livermore National Laboratory by Drs. Rod Balhorn, Andrew Wyrobek and Larry Stanker and have been used by researchers worldwide since the 1980’s to identify mammalian protamines, monitor their expression in spermatids and detect their presence in mature sperm.
Our products are sold for research or laboratory use only. They are not to be administered to humans. Resale for in vivo diagnostic, therapeutic or drug discovery purposes is prohibited; a license from Lawrence Livermore National Laboratory is required for these purposes
Antigen Selectivity
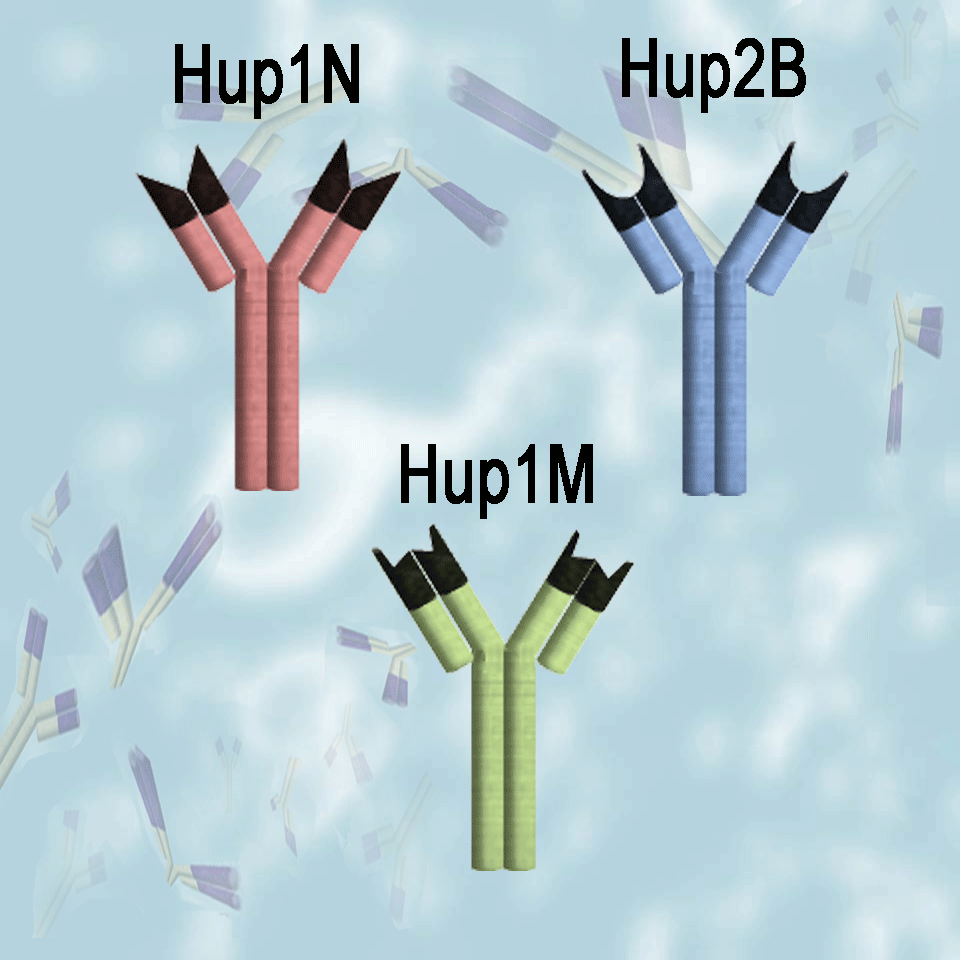
Hup1N, Hup2B and Hup1M were generated in mice using the native protamine molecules and chromatin particles isolated from mature human sperm as antigens. They are provided as affinity purified reagents. These antibodies do not cross react with oligo-arginine or histone
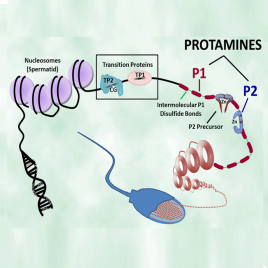
Hup 1N is an IgG1 antibody that binds selectively to the protamine P1 molecules present in the spermatids and sperm of a wide variety of mammalian species, including human, Cynomologus monkey, Rhesus monkey, boar, bull, dog, mule deer, ram, chinchilla, Rattus norvegicus ,cotton rat, gerbil, mouse, and Syrian hamster. Hup1N does not react well with protamine P1 from Voles (Microtus species) or rabbits. The epitope has been localized to the amino terminus of the protein (first six amino acids).
Hup 2B is an IgG1 antibody that binds selectively to both the fully processed (mature) and precursor protamine P2 molecules isolated from a wide variety of mammals, including human, mouse, certain Rattus species, and Syrian hamster. The epitope has been localized to a segment in the first half of the fully processed P2 molecule.
Hup 1M is an IgG1 antibody that is highly specific and binds only to human protamine P1 molecules. Its epitope is not known.
Antibody Concentrations
- Hup1N and Hup2B: 2.1 mg/ml in PBS, pH 7.4
- Hup1M: 1.3 mg/ml in PBS, pH 7.4
Volumes Provided
- Hup1N and Hup2B: 150 µg size contains 80 µl, 300 µg size contains 160 µl, 1000 µg size contains 533 µl
- Hup1M:150µg size contains 130 µl, 300 µg size contains 260µl, 1000 µg size contains 867 µl
Recommended Dilutions
The best antibody dilution for a particular application is generally determined by the user and depends on the concentration and availability of the antigen, concentration of the antibody, the type of antibody tag and the method that will be used to detect or visualize antibody binding. The following dilutions are typical examples that have been used by various investigators and should only be used as guidelines.
Storage
These antibodies should be stored at 4 °C if they are to be used within a month after their arrival. Freeze aliquots at -20 °C if they need to be stored for longer periods of time (up to 1 year). As with all antibodies, it is important to avoid subjecting the antibodies to repeated cycles of freezing and thawing.
Shipping
Unless otherwise specified, we will ship the antibodies on frozen gel pack. Shipping by FedEx overnight on 10 lbs (5 kg) dry ice for addition cost is also an option. Please note that we cannot ship on dry ice to certain countries. Please check your country’s rules and regulations before choosing this option. The antibodies are stable at ambient temperatures for several days. If they should arrive defrosted, aliquot into smaller portions. Keep the portions in current use in the refrigerator and freeze the rest. As with any antibody, please avoid REPEATED freeze thaw cycles.
Many customers prefer to use their own institutional shipping account because they have larger discounts, and/or to help speed an order through customs. If you have a shipping account number that you prefer to use, please click on that option in the checkout page and provide the type of service (e.g. FedEx, UPS, or DHL) and account number, or send us an email (support@briarpatchbio.com) or fax (1-925-215-2142) with this information. Your shipping account will be charged.
Protocols
See additional protocols on Useful Information Page. Note: Protamines are very basic and insoluble in SDS. Protamines cannot be run on standard SDS gels. Please see protocol for acid-urea gels instead.
Staining Protocol for Spermatids and Sperm Cells using Anti-protamine Antibodies
[Adapted from: Aoki, VW, Emery, BR, Liu, L, Carrell, DT. Protamine levels vary between individual sperm cells of
infertile human males and correlate with viability and DNA integrity. J Androl 27: 890 (2006).]
- The semen sample (or suspension of isolated spermatids) is washed in Phosphate Buffered Saline (PBS), pH 7.4, and the sperm/spermatids are pelleted by centrifugation.
- The pellets are resuspended in a small volume of PBS and a drop is smeared onto a pre-cleaned glass slide and examined to evaluate the density of sperm/spermatids spread across the slide. The suspension may need to be diluted to yield smears with appropriate sperm/spermatid densities on the slide.
- The slide is allowed to air dry at ambient temperature for a minimum of 1 hour.
- To ensure antibody accessibility to the nuclear proteins, the sperm/spermatid nuclei are decondensed slightly by incubating the slides in 10 mM DTT, 0.1M Tris buffer pH 8, followed by a 2-hour incubation in a solution containing 10 mM lithium diiodo-salicylate (LIS) and 1 mM DTT in 0.1 M Tris buffer.
- The slide containing the sperm/spermatid cells is then fixed in 4% paraformaldehyde in PBS, pH 7.4 for 1 hour.
- Following fixation, the slide is rinsed in PBS and the cells are permeabilized by treatment with 2% Triton X-100 and 0.1% bovine serum albumin in PBS for 15 minutes.
- The slide is incubated overnight with a 1/500 dilution of the primary antibody (Hup1M, Hup1N or Hup2B).
- The slide is then washed in PBS containing 2% Triton X-100 (15 min)
- The slide is subsequently incubated with a secondary antibody (e.g. goat antimouse antibody diluted 1/1000) tagged with a fluorochrome or other tag at ambient temperature overnight.
- If the secondary antibody is biotinylated, an additional step is required to treat the slide with the desired dye-tagged form of streptavidin as per instructions from the manufacturer.
- As a final step, the slide is rinsed to remove unbound secondary antibody and then viewed under the microscope.
|
Protocol for Immunostaining Fixed and Paraffin Embedded Tissue Sections
Note: This protocol is designed for staining formalin fixed, paraffin embedded tissue sections.
- Incubate slides in a dry oven at 60°C for 1 hour. Slides should be maintained in a vertical orientation to allow complete removal of the paraffin. Allow the slide to cool to room temperature.
- Deparaffinize by immersing the slides in xylene (5 times for 4 min).
- Hydrate slides by immersing the slides in 100%, 95%, 70% ethanol, 30% ethanol (2 times for 3 min each), and distilled or deionized water for 5 min.
- Before incubation with the antibodies, antigen retrieval must be performed. Immerse the slides in citrate buffer (0.01M, pH 6.0).
- Microwave (700W or high) for 5 min, add citrate buffer if necessary
- Microwave on medium for 5 min, add citrate buffer if necessary.
- Microwave on low for 5 min.
- After cooling, transfer to PBS (pH 7.4; 2 times for 5 min each),
- Incubate sections with 3% hydrogen peroxide for 20 minutes to eliminate endogenous peroxidase activity.
- Rinse sections with PBS (5 times for 2 min each).
- Incubate sections with 10% normal horse serum (Vector Laboratories) for 20 min in a humidified chamber at room temperature.
- Treat with primary antibody (diluted 1/500 in PBS) and allowed to sit overnight at 4 °C.
- Rinse sections with PBS (3 times for 5 min each).
- Treat slide with a biotinylated secondary anti-mouse antibody (2 hrs to overnight) and then rinse in PBS (3 times for 5 min each).
- Incubate sections with the chromogen DAB (from Vectastain ABC Elite kit, Vector Laboratories) for 2-10 min, depending on the intensity of staining desired.
- Stop the reaction by washing in distilled water.
- Counterstain slides in Mayer’s hematoxylin for 10 sec.
- Dehydrate slides in 30%, 70%, 95%, and 100% ethanol.
- Clear slides in xylene (4 times for 5 min each).
- Mount cover slide with Permount.
- View through a microscope.
|
Immunostaining of Western Blots using Anti-protamine Antibodies[Protocol Developed by Michele Corzett, Lawrence Livermore National Laboratory]Note: Wear powderless gloves when handling the transfer membranes
- Prepare a blocking solution containing the following (this is enough solution for 4 blots): 5 gm bovine serum albumin (Fraction V, RIA grade), 100 ml Solution A (0.M Tris pH 7.4, 0.1% TWEEN 20, 0.9% sodium chloride) and 200 µl calf thymus DNA (10 mg/ml) in water.
- Turn on and set hybridization oven at 37 °C.
- Wet the Immobilon-P membrane gel transfer with methanol and rinse the blot with water.
- Place the membrane in a 50 ml disposable plastic screw cap tube, add 25 ml of blocking solution, cap the tube, and place it in the hybridization oven at 37 °C for one hour with rotation
- Remove the tubes from the oven, cool to room temperature, and discard the blocking solution.
- Wash each membrane while in the tube with 40 ml of Solution A; repeat two additional times and drain thoroughly.
- Add 10µl of the primary antibody (Hup 1M, Hup 1N or Hup 2B) to 10 ml Solution A and transfer this to the tube containing the membrane. Return the tube to the hybridization oven and incubate at ambient temperature for 2 hr with rotation.
- Wash the membrane (while in the tube) with 40 ml Solution A, repeat two additional times, and then add 10 ml fresh Solution A to the tube and membrane.
- Add 35 µl biotinylated anti-mouse IgG secondary antibody to the tube and return to the hybridization oven for 30 min at room temperature with rotation.
- Prepare the substrate immediately and let sit for 30 min. The substrate contains 10 ml Solution A, 2 drops Reagent A and 2 drops Reagent B from an ABC-AP kit (Vector Laboratories).
- Wash the membrane (while in the tube) with 40 ml Solution A, repeat two additional times, and then add the 10 ml of the ABC-AP solution to the tube and membrane. Return to the oven (at RT) for 30 min with rotation.
- Wash the membrane (while in the tube) with 40 ml Solution B (0.1M Tris pH 7.4, 0.9% sodium chloride) and repeat two additional times.
- Prepare 10 ml of Vector Red substrate (Vector Laboratories) containing 10 ml 100mM Tris pH8, 4 drops Reagent 1, 4 drops Reagent 2 and 4 drops Reagent 3 as described in the instructions provided with the reagents and add to the tube and membrane. Invert the tube to flow substrate over the membrane until the color begins to develop.
- Transfer the membrane to a petri dish and watch until color is fully developed (but before the background becomes too dark). Pour off the subtrate and wash the membrane in water for 5 min. Photograph.
- To probe with a second primary antibody, re-wet the membrane with methanol, rinse with water and add the primary antibody (step 7). There is no need to reblock. Continue as with first antibody, but use the Vector Blue substrate to visualize the second antibody.
|
Transfer of Protamines from Acid-Urea Gels to Immobilin-P (Western Blot)
[Protocol Developed by Michele Corzett, Lawrence Livermore National Laboratory]
Note: Wear powderless gloves when handling the gels and transfer membranes
- Place enough transfer solution (0.0009N acetic acid) in a clean photographic tray to cover the bottom of the tray.
- Wet two pieces of Immobilon-P membrane (Millipore) for each gel to be transferred (precut to size) with methanol and place in the transfer solution.
- Soak two precut pieces of filter paper and three scotchbrite pads (by squeezing) in transfer solution for each gel to be transferred.
- After unloading the gel from the cassette, mark lane 1 by punching holes in the gel above the lane, cut off the thick area on the bottom of the gel, and soak the gel in transfer solution for 10 min.
- Assemble the following stack of pads, filter paper, membrane and gel in the following order, keeping everything wet and without introducing air bubbles:
- Scotchbrite
- Filter paper
- Gel (hole on left side)
- Immobilon-P membrane (with notch cut out on left side)
- Second piece of Immobilon-P membrane
- Filter paper
- Scotchbrite
If transferring two gels at the same time, continue the stack:
- Filter paper
- Gel #2 (two holes on left side)
- Immobilon-P membrane (with two notches cut out on left side)
- Second piece of Immobilon-P membrane
- Filter paper
- Scotchbrite
- Insert the gel/membrane/filterpaper/scotchbrite ‘sandwich’ in the transfer holder carefully to prevent the layers from slipping. If needed, add more scotchbrites on the outside of the ‘sandwich’ to get a tight fit in the holder and apply the top to the transfer holder.
- Fill the gel box to half full with transfer solution and place the transfer holder with the gel ‘sandwich’ in the gel box. Attach the electrodes and confirm they are in the right orientation to move the protamines toward the Immobilon-P membrane.
- Fill the gel box with transfer solution until the gel is totally covered.
- Turn on power and perform transfer at 50V for 15 min.
- Disassemble the ‘sandwich’, remove the membrane(s) with the notches, place on white absorbent paper towels and let dry.
|